The Clinical Trails of Nuedexta Medication
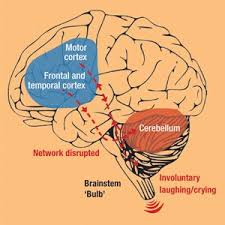
Nuedexta is a drug which is being used to treat people who are suffering from a condition known as Pseudobulbar affect. Nuedexta is still quite a young, and untested drug, and there are a series of clinical trials being performed at the moment to determine the effectiveness and safety of the drug.
The Nuedexta clinical trials have, so far, been quite positive and promising. It should not be long before the drug is prescribed commonly to treat people with PBA. There are always safety concerns with new medications, and the outcomes of the clinical trials so far are being watched quite closely.
Nuedexta Has Been Licensed for Marketing in Some Parts of the World
Neudexta was given a marketing license in 2013 by the EMA. The license was withdrawn for EU marketing in 2016 and did not get licensed at all for marketing in the UK. For it to be licensed, it will need far more testing than it has had so far. The drug is still available in the USA.
Effects of Nuedexta Studied in Clinical Trials
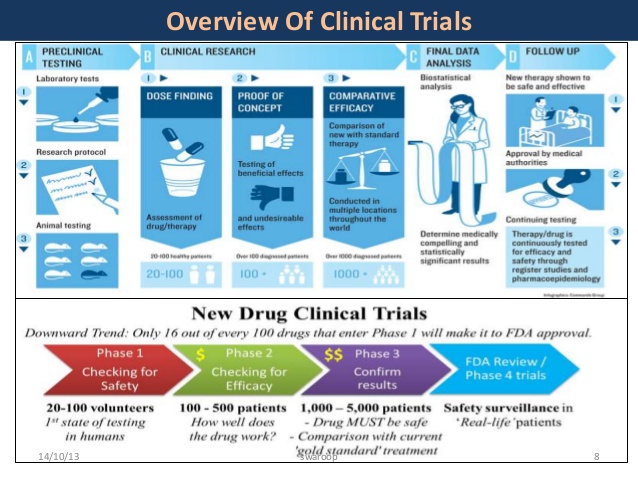
One of the most recent Nuedexta clinical trials focused on the effects of the drug on nursing home patients who suffered from PBA. The experiment spanned six months and looked at a total of 125 patients across several states. Unfortunately, evidence from this study was inconclusive because there was difficulty recruiting patients, so the study was terminated before the six month period was up.
Another study looked at the safety and tolerability of Nuedexta for managing PBR in people who had suffered from a range of conditions, including traumatic brain injury and dementia, as well as strokes. This study spanned 12 weeks and used a 20mg dose of Nuedexta. The study engaged 367 participants and showed promising results across several outcome measures relating to emotional episodes and other difficulties.
Nuedexta is Often Mis-Prescribed

Clinical trials have been promising when Nuedexta has been tested for PBA alone. However, there are some concerns about the safety of the drug and the way that it is prescribed. Recent research and studies into the prescription of the medication indicate that doctors often prescribe it to people who have dementia, Parkinson’s, or another underlying condition. This is concerning because the drug has not been tested for use in this way. It is uncertain whether there may be interactions between Nuedexta and other drugs, or whether it may have unexpected impacts on people who are suffering from other conditions. This is something that the medical community is calling for further research.
Technically, prescribing Neudexta for PBA that is associated with other underlying conditions is off-label. This is something that a lot of doctors and consultants overlook, but it should be a matter of significant concern given the potential for the drug to have unwanted side-effects.
Indeed, Medicare has asked that insurance companies monitor the prescription of Nuedexta. That’s because it is being marketed aggressively for dementia patients. Though, it has not been properly tested and trialed for that kind of use. It is also being marketed for Alzheimer’s. Other drugs are likely to be far better suited for managing the symptoms that people living with Alzheimer’s are experiencing. People who use Nuedexta for Alzheimer’s are more likely to experience falls, and other secondary issues, including UTIs, than those who are given more appropriate drugs.
One challenge that doctors face is that there are not many options for managing the behavioral issues associated with Alzheimer’s and dementia. It can be tempting to use something like Nuedexta, which may help with those issues in the short term. Unfortunately, Nuedexta is just a band-aid for such problems, and it is not going to manage the condition itself. In the vast majority of cases, it would be better for doctors to find ways to proactively slow the progress of Alzheimer’s or to find ways for the patient to cope with dementia without using an off-label drug that has not been tested for that kind of application. Doctors and nursing home workers are encouraged to avoid this kind of excessive over-prescription until more data is clarifying the safety of the drug.







 Under this patient assistance program, the eligible individuals that have ALS can receive a six-month supply of Nuedexta for free and can also qualify for an ongoing assistance that includes periodic verification in regards to eligibility.
Under this patient assistance program, the eligible individuals that have ALS can receive a six-month supply of Nuedexta for free and can also qualify for an ongoing assistance that includes periodic verification in regards to eligibility.


 Some people have also had a difficulty with weight gain when taking Nuedexta, but it does not seem to be a common problem. This may be an issue that is directly associated with taking this drug, but it may also be due to a combination of medications that are prescribed by your physician or perhaps even one of the other medications that you are currently taking. This is also a side effect that you should be discussing with your doctor if it occurs, as there may be ways to control the problem or to overcome it.
Some people have also had a difficulty with weight gain when taking Nuedexta, but it does not seem to be a common problem. This may be an issue that is directly associated with taking this drug, but it may also be due to a combination of medications that are prescribed by your physician or perhaps even one of the other medications that you are currently taking. This is also a side effect that you should be discussing with your doctor if it occurs, as there may be ways to control the problem or to overcome it.
 There are some
There are some 
